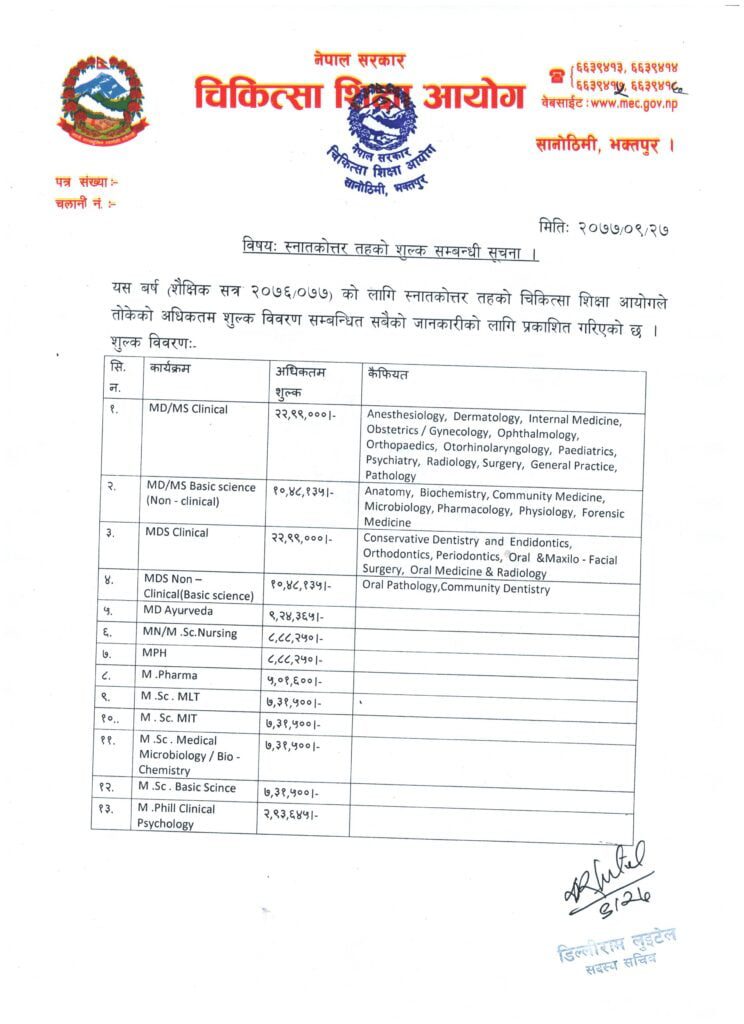
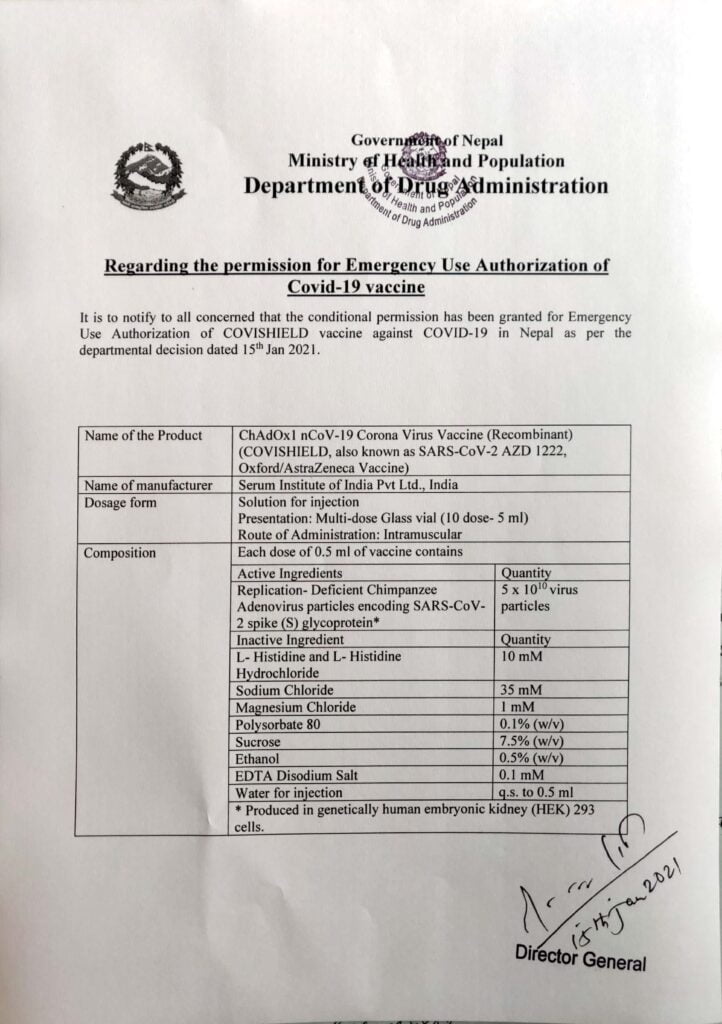
The Department of Drug Administration (DDA) has decided to approve ‘COVISHIELD’ COVID-19 vaccine for emergency use in Nepal. DDA provided the conditional permission for emergency authorisation of COVISHEILD vaccine against COVID-19. COVISHEILD is the first COVID-19 vaccine approved for use in Nepal.
Detail notice;
Related
- What is COVAX? Why we need COVAX? What COVAX offers?
- Principles for sharing COVID-19 Vaccine doses with COVAX
- Vaccines development process & Clinical trials
- World Immunization Week 2020 #VaccinesWork for All
Do you have a website? Looking for the best hosting provider? Here’s a discount code.
Latest Public Health Jobs
Latest Posts
- Multisectoral Action Plan for the Prevention and Control of NCDs, 2026-2030 (Draft)
- National Standard Operating Procedure for Early Warning, Alert and Response System (EWARS), 2025
- Priority Infectious Diseases for Community-Based Surveillance in Nepal
- Community Based Disease Surveillance Guideline, 2082
- Political declaration of the fourth high-level meeting of the General Assembly on the prevention and control of NCDs and the promotion of mental health and well-being
Thanks for visiting us.
Disclaimer: The resources, documents, guidelines, and information on this blog have been collected from various sources and are intended for informational purposes only. Information published on or through this website and affiliated social media channels does not represent the intention, plan, or strategies of an organization that the initiator is associated with in a professional or personal capacity, unless explicitly indicated.
If you have any complaints, information, or suggestions about the content published on Public Health Update, please feel free to contact us at blog.publichealthupdate@gmail.com.
#StayUpdated