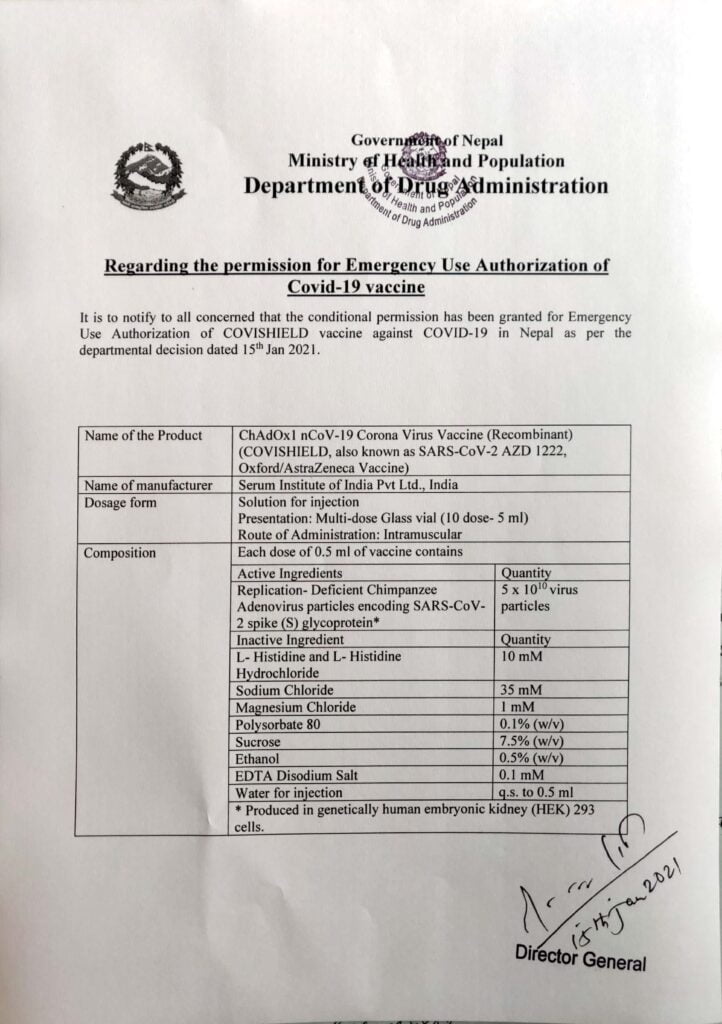
The Department of Drug Administration (DDA) has decided to approve ‘COVISHIELD’ COVID-19 vaccine for emergency use in Nepal. DDA provided the conditional permission for emergency authorisation of COVISHEILD vaccine against COVID-19. COVISHEILD is the first COVID-19 vaccine approved for use in Nepal.
Detail notice;
Related
- What is COVAX? Why we need COVAX? What COVAX offers?
- Principles for sharing COVID-19 Vaccine doses with COVAX
- Vaccines development process & Clinical trials
- World Immunization Week 2020 #VaccinesWork for All
Do you have a website? Looking for the best hosting provider? Here’s a discount code.
Latest Public Health Jobs
Latest Posts
- Salim Yusuf Emerging Leaders Programme 2026
- Global curriculum guide for community health workers
- Australia Awards Scholarships 2027
- APO Health systems reviews template for authors
- Research Fellowship in Public Health Intelligence – Berlin 2026
Thanks for visiting us.
Disclaimer: The resources, documents, guidelines, and information on this blog have been collected from various sources and are intended for informational purposes only. Information published on or through this website and affiliated social media channels does not represent the intention, plan, or strategies of an organization that the initiator is associated with in a professional or personal capacity, unless explicitly indicated.
If you have any complaints, information, or suggestions about the content published on Public Health Update, please feel free to contact us at blog.publichealthupdate@gmail.com.
#StayUpdated



Comments are closed.