Preclinical phase
Each vaccine under development must first undergo screenings and evaluations to determine which antigen should be used to invoke an immune response. This preclinical phase is done without testing on humans.
An experimental vaccine is first tested in animals to evaluate its safety and potential to prevent disease.
If the vaccine triggers an immune response, it is then tested in human clinical trials in three phases.
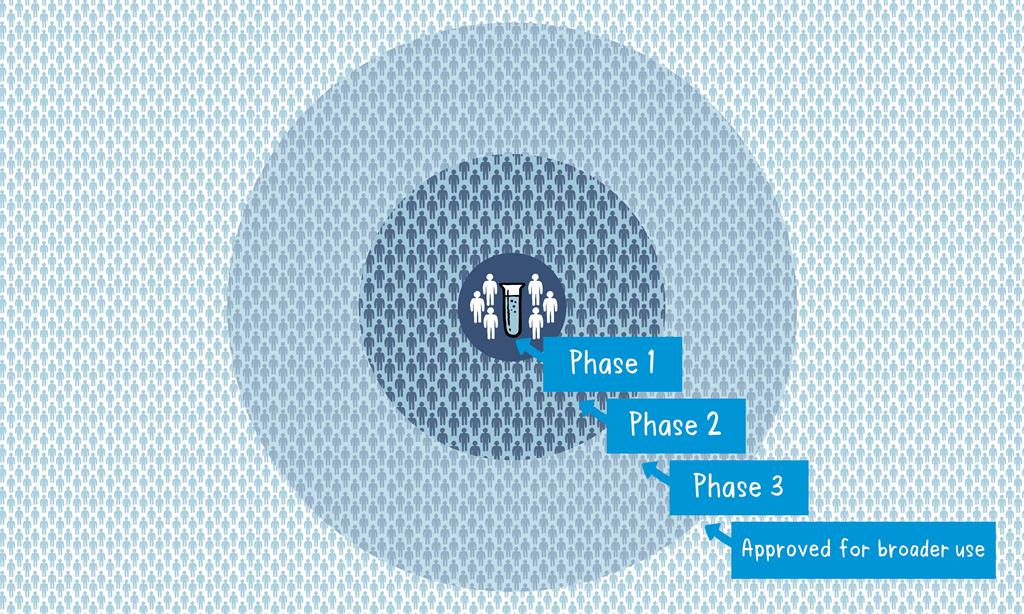
Phase 1
The vaccine is given to a small number of volunteers to assess its safety, confirm it generates an immune response, and determine the right dosage. Generally in this phase vaccines are tested in young, healthy adult volunteers.
Phase 2
The vaccine is then given to several hundred volunteers to further assess its safety and ability to generate an immune response. Participants in this phase have the same characteristics (such as age, sex) as the people for whom the vaccine is intended. There are usually multiple trials in this phase to evaluate various age groups and different formulations of the vaccine. A group that did not get the vaccine is usually included in phase as a comparator group to determine whether the changes in the vaccinated group are attributed to the vaccine, or have happened by chance.
Phase 3
The vaccine is next given to thousands of volunteers – and compared to a similar group of people who didn’t get the vaccine, but received a comparator product – to determine if the vaccine is effective against the disease it is designed to protect against and to study its safety in a much larger group of people. Most of the time phase three trials are conducted across multiple countries and multiple sites within a country to assure the findings of the vaccine performance apply to many different populations.
Blinding
During phase two and phase three trials, the volunteers and the scientists conducting the study are shielded from knowing which volunteers had received the vaccine being tested or the comparator product. This is called “blinding” and is necessary to assure that neither the volunteers nor the scientists are influenced in their assessment of safety or effectiveness by knowing who got which product. After the trial is over and all the results are finalized, the volunteers and the trial scientists are informed who received the vaccine and who received the comparator.
Next step
When the results of all these clinical trials are available, a series of steps is required, including reviews of efficacy and safety for regulatory and public health policy approvals.
Officials in each country closely review the study data and decide whether to authorize the vaccine for use. A vaccine must be proven to be safe and effective across a broad population before it will be approved and introduced into a national immunization programme. The bar for vaccine safety and efficacy is extremely high, recognizing that vaccines are given to people who are otherwise healthy and specifically free from the illness.
Further monitoring takes place in an ongoing way after the vaccine is introduced. There are systems to monitor the safety and effectiveness of all vaccines. This enables scientists to keep track of vaccine impact and safety even as they are used in a large number of people, over a long time frame. These data are used to adjust the policies for vaccine use to optimize their impact, and they also allow the vaccine to be safely tracked throughout its use.
Once a vaccine is in use, it must be continuously monitored to make sure it continues to be safe.
Source of info: WORLD HEALTH ORGANIZATION
Recommended readings
- VACCINES DEVELOPMENT PROCESS & CLINICAL TRIALS
- Call to Action: Vaccine Equity Declaration
- WHO lists two additional COVID-19 vaccines for emergency use and COVAX roll-out
- COVID-19 Vaccine FAQs (Nepali)
- DDA approves ‘COVISHIELD’ vaccine for emergency use in Nepal
- Orientation to National Deployment and Vaccination Planning for COVID-19 Vaccines
- WHO issues its first emergency use validation for a COVID-19 vaccine
- Principles for sharing COVID-19 Vaccine doses with COVAX
- Online Course: Vaccine Economics Online Course
- WHO convenes manufacturers, regulatory authorities meet on COVID-19 vaccines
- WHO ADDS JANSSEN VACCINE TO LIST OF SAFE AND EFFECTIVE EMERGENCY TOOLS AGAINST COVID-19
Do you have a website? Looking for the best hosting provider? Here’s a discount code.
Latest Public Health Jobs
Latest Posts
- National workforce capacity for essential public health functions: Operational handbook for country-led contextualization and implementation
- World Conference on Lung Health Travel Grants 2025
- GIS Training Manual for Health Statistics
- WHO launches bold push to raise health taxes and save millions of lives
- National Oral Health Strategy 2081-2087
Thanks for visiting us.
Disclaimer: The resources, documents, guidelines, and information on this blog have been collected from various sources and are intended for informational purposes only. Information published on or through this website and affiliated social media channels does not represent the intention, plan, or strategies of an organization that the initiator is associated with in a professional or personal capacity, unless explicitly indicated.
If you have any complaints, information, or suggestions about the content published on Public Health Update, please feel free to contact us at blog.publichealthupdate@gmail.com.
#StayUpdated



Comments are closed.