Overview
The Ministry of Health and Population (MoHP) Nepal has released new guidelines on the National Antimicrobial Treatment Guidelines 2023. The National Antibiotic Treatment Guideline was initially released by the MoHP in 2014.
The new guidelines aimed to address the emergence and spread of resistance, optimize the use of available antimicrobial agents, reduce selection pressure through appropriate control measures, change the behavior of prescribers and communities to ensure rational use, and combat AMR through nationally coordinated efforts. The updated version of the National Antimicrobial Treatment Guidelines aims to provide prescribers with the necessary guidance on selecting the right drug, dose, and duration for commonly encountered infectious conditions in Nepal.
The document incorporates the cumulative antibiogram from national AMR surveillance data, considers the availability and affordability of drugs in Nepal, references the National List of Essential Medicines (NLEM), and consults other national and international protocols and guidelines. Efforts have been made to prioritize antibiotics from the “Access” group (as per the WHO AWaRe Classification of antibiotics) as the first-line therapy whenever possible. It should be noted that this document adopts the WHO’s AWaRe classification.
Scope of the guideline
- This document provides information to healthcare workers on the rational use of antibiotics for empirical or definitive treatment of commonly encountered infections in Nepal, as well as various prophylactic measures. However, it is not exhaustive and excludes infections for which national treatment protocols already exist.
- This document will be regularly updated as new data becomes available.
Objectives
- To offer guidance for the optimal use of antimicrobials in various infectious conditions and for prophylactic purposes, taking into account the cumulative antibiogram from National AMR surveillance.
- To promote the preferential use of antimicrobials from the “Access” group, while ensuring judicious use of those from the “Watch” and “Reserve” groups.
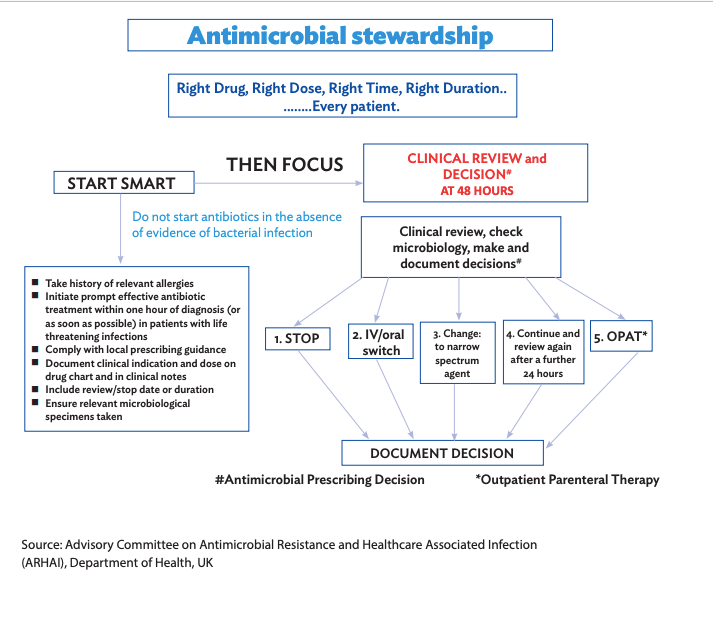
General Principles
Empirical Therapy – Antibiotic treatment is considered empirical when it is administered in the absence of microbiological confirmation or while awaiting pending reports. Reevaluation of empirical antibiotics must be conducted after 48-72 hours and once the reports become available.
Important considerations
Determine if antibiotic therapy is necessary. Discontinue if the cause is determined to be non-infectious. Seek assistance
from the Antimicrobial Stewardship (AMS) team within the institution, if available.
Evaluate the possibility of using a narrow-spectrum antibiotic based on the reports. Consider de-escalating the antibiotics based on the clinical condition and available reports.
- Assess if switching to monotherapy is appropriate (if initially using a combination).
- Evaluate the feasibility of changing the route of administration to oral.
- Adjust the dosage based on renal and hepatic functions, if necessary.
- Check for potential drug interactions with other medications being used.
- Determine if any laboratory parameters need monitoring during therapy.
These recommendations serve as a treatment guide and do not replace the clinical judgment of the responsible physician after a comprehensive assessment of each individual case.
Prescriptions should clearly include
- Indication for antibiotic use.
- Formulation; Capsule/Tablet or Injection.
- Route of administration (e.g., IM or IV), infusion rate for IV, and dosing.
- Start date, review date, stop date, or duration.
Consider implementing transmission-based precautions and isolation for patients with infectious diseases and drugresistant organisms such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), drug-resistant tuberculosis (DRTB), Clostridioides difficile, etc.
Precautions to observe
- Standard precautions: Practice proper hand hygiene, respiratory hygiene, sharps safety, safe injection practices, use sterile instruments and devices, maintain clean and disinfected environmental surfaces, and use gloves and protective clothings. Use mouth, nose, and eye protection during procedures.
- Contact precautions: Ensure appropriate patient placement, limit patient transport, use disposable or dedicated equipment, perform cleaning and disinfection of the room, and use gloves gown.
- Droplet precautions: Have patients wear masks for source control, ensure appropriate patient placement, limit patient transport, and provide masks for healthcare personnel.
- Airborne precautions: Ensure patients wear masks for source control, place them in isolation rooms, limit patient transport, restrict susceptible healthcare personnel from entering the room, and have healthcare personnel wear N-95 masks or higher level respirators.
Please note that these precautions are subject to local guidelines and protocols and may require additional measures based on the specific circumstances.
- World Obesity Day 2026 | 8 Billion Reasons to Act on Obesity
- Salim Yusuf Emerging Leaders Programme 2026
- Global curriculum guide for community health workers
- Australia Awards Scholarships 2027
- APO Health systems reviews template for authors


