Government of Nepal nominated Department of Drug Administration (DDA) in October 2004 as the focal point (National Pharmacovigilance centre) to liaison with WHO collaborating centre for International Drug Monitoring, Sweden and started collecting adverse drug reactions. Nepal became a WHO programme member in July 2006.
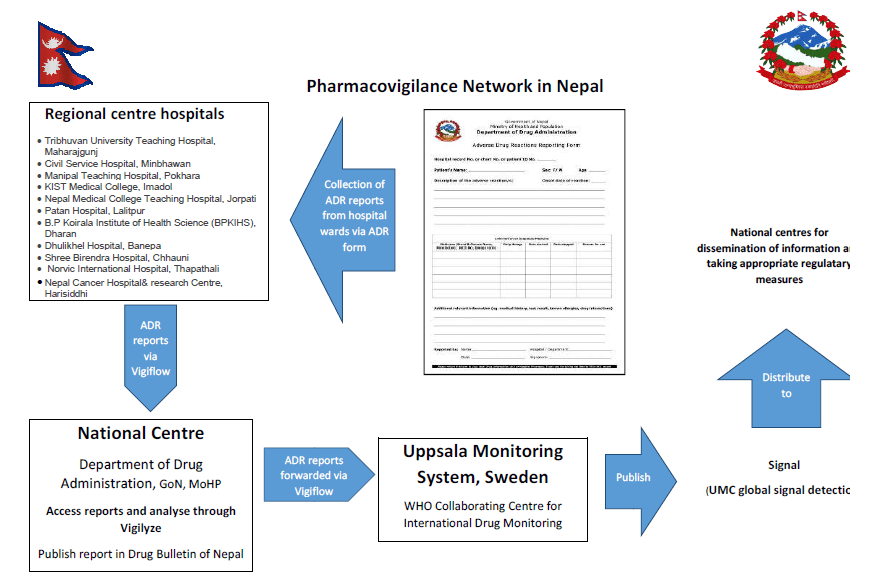
At present, there are 15 regional pharmacovigilance centers in Nepal
- Tribhuvan University Teaching Hospital, Maharajgunj
- Civil Service Hospital, Minbhawan
- Manipal Teaching Hospital, Pokhara
- KIST Medical College, Imadol
- Nepal Medical College Teaching Hospital, Jorpati
- Patan Hospital, Lalitpur
- B.P Koirala Institute of Health Science (BPKIHS), Dharan
- Dhulikhel Hospital, Banepa
- Shree Birendra Hospital, Chhauni
- Norvic International Hospital, Thapathali
- Nepal Cancer Hospital and Research Center, Harisiddhi
- College of Medical Sciences – Teaching Hospital
- Nepal Mediciti, Kathmandu.
- Chitwan Medical College, Chitwan.
- National Tuberculosis Control Center, Bhaktapur.
These regional pharmacovigilance centers operate under DDA (DDA being the National centre for ADR monitoring). The regional centers reports ADRs to the National center (DDA) via ‘Vigiflow’ (an online program) which are then forwarded to the Uppsala Monitoring Center (UMC) by the National Centre. The National database contains about 972 ADR reports so far.
Source of Info: DDA Website
Related readings
- Department of Drug Administration (DDA)
- List of Domestic Industries listed in DDA DAMS
- WHO GMP Certified Pharmaceutical Companies in Nepal
- List of Narcotics and Psychotropic Substances identified for Import and Use in Nepal
- Pharmacovigilance Network in Nepal
- Drug and Medicine Related Act, Rules, Regulations, Policies, Guidelines & Directives in Nepal
- New Drug Registration Process & Format of Documents for Import in Nepal


